Percent Yield Definition Chemistry
Famous Percent Yield Definition Chemistry Ideas. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. Measuring the amount of product formed gives us the actual yield.
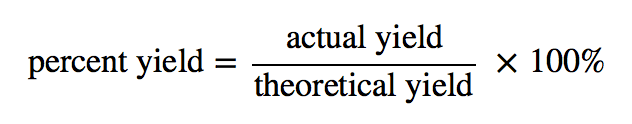
The actual yield is the amount that was actually made, which was 65.2 g of zn (no 3) 2. In chemistry, percent yield is a comparison of actual yield to theoretical yield, expressed as a. This gives us a percentage of what we have expected, which in chemistry we call the percentage yield.
This Gives Us A Percentage Of What We Have Expected, Which In Chemistry We Call The Percentage Yield.
\[percentage~yield = \frac{yield~obtained}{theoretical~yield} \times 100\] for example, if the predicted yield is 20 g. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as. It is the amount of product resulting.
Mgco 3 → Mgo + Co 2.
Meaning and definition of percent yield : Copper oxide reacts with sulfuric acid to make copper sulfate and. But the question states that the actual yield is only 37.91 g of sodium sulfate.
The Percentage Yield Can Vary From 100% (No Product Has Been Lost) To 0% (No Product Has Been Made).
Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated, multiplied by 100%. % yield = actual yield theoretical yield ⋅ 100%. The actual yield of a product as a percentage of the theoretical yield.
Which Means The Actual And Theoretical Yield Have To Have The Same Units When You Divide Them.
The percent yield, therefore, tells the chemist about the success of the reactions and it is a ratio between the actual and the theoretical yield and is expressed in the form of a percentage. Measuring the amount of product formed gives us the actual yield. Yield (chemistry) in chemistry, yield, also referred to as reaction yield, is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a.
Give The Part Of The Percent Yield.
The percent yield has no units. The above reaction shows that for. The actual yield is commonly represented as a percent yield, indicating how close the actual yield was to the anticipated.
Post a Comment for "Percent Yield Definition Chemistry"